Kicking off with Pfizer Side Effects Monitoring and Safety Reporting, this opening paragraph is designed to captivate and engage the readers, setting the tone for a comprehensive discussion on the importance of monitoring side effects and safety reporting in the context of Pfizer vaccines.
As we delve into the mechanisms used by Pfizer, the role of healthcare providers, and the significance of community engagement, the complexity of ensuring vaccine safety becomes clearer.
Importance of Monitoring Side Effects
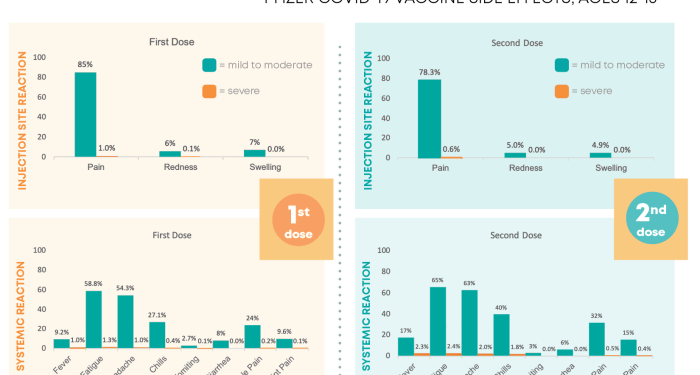
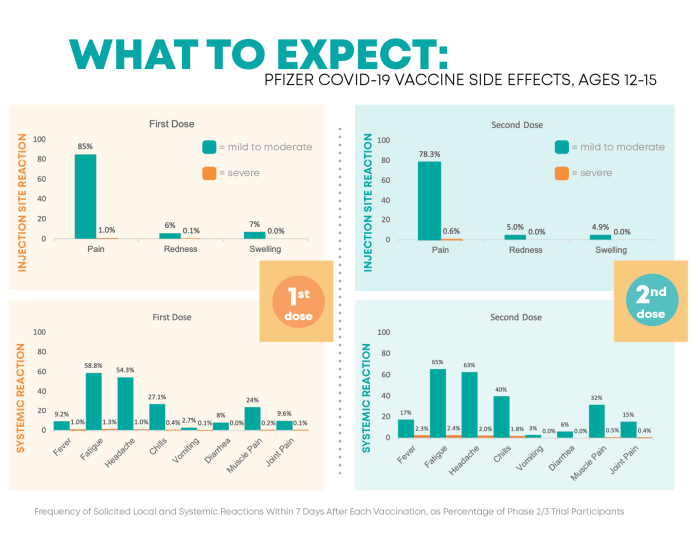
Monitoring the side effects of Pfizer vaccines is crucial in ensuring the safety and efficacy of the vaccination program. By closely tracking and reporting any adverse reactions, healthcare professionals can promptly address any potential concerns and prevent serious complications.
Potential Side Effects That Need Close Monitoring
- Injection site reactions such as pain, redness, or swelling
- Flu-like symptoms including fever, chills, and fatigue
- Allergic reactions like rash, itching, or difficulty breathing
- Gastrointestinal issues such as nausea, vomiting, or diarrhea
Impact of Timely Reporting on Public Health and Safety
Timely reporting of side effects is essential for maintaining public trust in the vaccination process and ensuring the overall safety of the population. By promptly addressing any adverse events, healthcare authorities can take necessary actions to protect individuals and prevent potential outbreaks.
This proactive approach can help mitigate risks and enhance the effectiveness of the vaccination campaign.
Pfizer Safety Reporting Systems
Pfizer employs robust mechanisms to monitor and report side effects of its medications to ensure the safety and well-being of patients. These systems are essential in detecting any potential adverse reactions and taking timely actions to mitigate risks.
Mechanisms Used by Pfizer
- Pfizer utilizes a global pharmacovigilance system that collects and analyzes data from various sources, including healthcare providers, patients, and clinical trials.
- The company employs advanced technologies and data analytics to identify potential safety signals and trends in real-time.
- Pfizer collaborates with regulatory authorities worldwide to ensure compliance with reporting requirements and to share safety information.
Comparison with Other Pharmaceutical Companies
- Pfizer's safety reporting systems are known for their thoroughness and transparency, setting a high standard in the pharmaceutical industry.
- Compared to other companies, Pfizer has a well-established infrastructure for monitoring and reporting side effects, enabling quick responses to emerging safety concerns.
- The company's proactive approach to safety reporting has earned it recognition for its commitment to patient safety.
Key Components of Pfizer’s Safety Monitoring Infrastructure
- Centralized pharmacovigilance database for tracking and analyzing adverse event reports.
- Dedicated team of safety experts responsible for evaluating safety data and implementing risk management strategies.
- Regular safety assessments and reviews to ensure ongoing monitoring of product safety profiles.
Healthcare Provider Role
Healthcare providers play a crucial role in monitoring and reporting Pfizer vaccine side effects to ensure the safety and well-being of patients. By actively monitoring and reporting adverse reactions, healthcare providers contribute to the ongoing evaluation of vaccine safety and effectiveness.
Best Practices for Healthcare Providers
- Encourage patients to report any side effects they experience after receiving the Pfizer vaccine.
- Stay informed about the latest guidance on vaccine safety and reporting requirements.
- Document and report all adverse reactions promptly through the appropriate channels.
- Provide education to patients about common side effects and when to seek medical attention.
- Collaborate with public health authorities to share important safety information and updates.
Successful Interventions by Healthcare Providers
- Early detection and reporting of rare side effects that led to swift regulatory action.
- Implementation of effective communication strategies to address vaccine safety concerns in the community.
- Participation in vaccine safety monitoring programs to contribute valuable data for ongoing assessment.
- Providing support and guidance to patients experiencing side effects, ensuring timely medical intervention when needed.
Community Engagement
Community engagement plays a vital role in monitoring the safety of the Pfizer vaccine. By involving the community, we can gather a broader range of feedback and reports on potential side effects, ensuring a more comprehensive understanding of the vaccine's safety profile.
Importance of Community Engagement
Engaging the community in monitoring Pfizer vaccine safety allows for a more transparent and inclusive approach to healthcare. It fosters trust and confidence in the vaccination process and encourages active participation in reporting any adverse reactions.
- Establishing community forums or advisory boards where individuals can share their experiences and concerns regarding the Pfizer vaccine.
- Collaborating with local community leaders, organizations, and healthcare providers to promote vaccine safety awareness and reporting.
- Utilizing social media platforms and online forums to encourage open discussions and information sharing about vaccine side effects.
Strategies for Involving the Community
Implementing strategies to involve the community in reporting side effects can enhance the overall safety monitoring process for the Pfizer vaccine.
- Conducting educational workshops and training sessions for community members on how to recognize and report potential side effects of the vaccine.
- Encouraging healthcare providers to actively engage with their patients and address any concerns or questions related to vaccine safety.
- Offering incentives or rewards for individuals who participate in vaccine safety reporting initiatives to increase community involvement.
Successful Community-Led Initiatives
There have been several successful community-led initiatives focused on monitoring vaccine safety, including those related to the Pfizer vaccine.
- The Vaccine Adverse Event Reporting System (VAERS) relies on community reports to track and investigate potential adverse reactions to vaccines, including the Pfizer vaccine.
- Local grassroots organizations partnering with healthcare providers to establish vaccine safety monitoring programs tailored to the needs of specific communities.
- Community-driven social media campaigns that raise awareness about the importance of reporting vaccine side effects and provide resources for individuals to share their experiences.
Last Word
In conclusion, Pfizer Side Effects Monitoring and Safety Reporting play a critical role in safeguarding public health, emphasizing the need for continuous vigilance and collaboration among various stakeholders.
FAQ Compilation
Why is monitoring side effects of Pfizer vaccines crucial?
Monitoring side effects is crucial to ensure the safety and efficacy of Pfizer vaccines, identifying any potential issues early on.
How do healthcare providers contribute to monitoring and reporting Pfizer vaccine side effects?
Healthcare providers play a key role in monitoring and reporting side effects, providing valuable data for safety assessments.
What are some successful community-led initiatives in vaccine safety monitoring?
Community-led initiatives like support groups and awareness campaigns have been successful in promoting vaccine safety.